先知大联考卷·2022届高三第二次大联考理科综合试题答案

先知大联考卷·2022届高三第二次大联考理科综合试题答案,目前我们趣对答案已经整理了先知大联考卷·2022届高三第二次大联考理科综合试题答案的各科答案和试卷,更多试卷答案请关注本趣对答案。


Paragraph IJatin looked up and he saw the other boys rushingahead. Refusing to give up at this crucial point, Jatinclenched his teeth, jumped to his feet and ran as fast as hecould. Finally, he was the fourth one to dash across thefinish line. Jatin couldnt help sobbing at the thought ofletting his sister down. But much to his surprise, the run-ner who collided with him was disqualified for breaking therule and Jatin won the third prize. Jatin felt overjoyed andrelieved during the award ceremony, holding the shoesclose to his chestParagraph 2Filled writh pleasure, Jatin walked home in no timeHe could not wait to see his sister and share the good newsOn his arrival, Jatin found Neha was walking about outsidnervously and anxiously. " Neha, see what I have got foryou.Jatin said excitedly, taking the prize out of his bagwith his trembling hands. The instant Neha saw the shoesher face lit up. So thrilled was she that she threw herself atJatin."Thank you! Jatin. " Tears of joy welled up in theieyes as the brother and the sister hugged tightly together
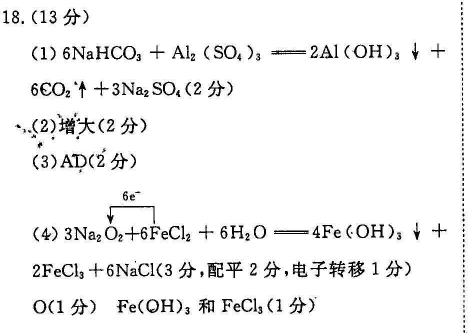

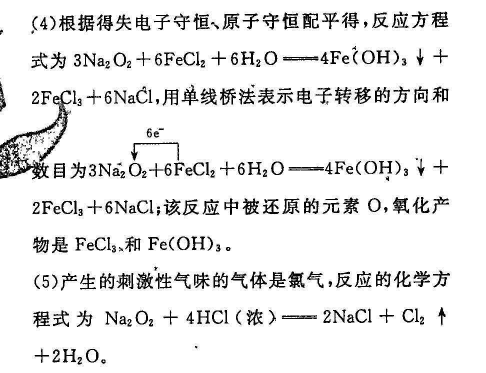
18.(13分)(1)6NaHCO3+Al2 (SO )3=2Al(OH)3+6CO2↑+3Na2SO4(2分),(2)增大(2分)(3)AD(2分)6e(4)3Na202+6FeCl2+6H20=4Fe(OH)3+2FeCl3+6NaCl(3分,配平2分,电子转移1分)O(1分)Fe(OH)3和FeCl3(1分)(5)Na2O2+4HCl(浓)=2NaCl+Cl2↑+2H2O(2分)【解析】(1) NaHCO3与Al2(SO4)3反应的化学方程式为Al2(SO4)3+6 NaHcO3=2Al(OH)3↓+6CO2↑+3Na2SO4。(2)Ba(OH)2少量,发生反应Ba21+20H+2HCO亠BaCO3↓+2H2O+CO%,溶液中c(CO3-)增大(3)滤出小苏打后,母液中含有NH4Cl和 NaHcO3,通入氨气,c(NH)增大,再加入食盐,使NHCl析出,然后过滤,NH4Cl的纯度较高,A项正确;②析出的NH4C中含有较多的NaCl,NH4Cl的纯度降低,B项错误;①中滤液还有较多的NH3,不能直接循环使用,C项错误;②中滤液含有较多NaCl,可以直接循环使用,D项正确。4)根据得失电子守恒原子守恒配平得,反应方程式为3Na2O2+6FeCl2+6H2O=4Fe(OH)3↓+2Fecl2+6NaCl,用单线桥法表示电子转移的方向和数目为3N2O2+6FeCl2+6H2O=4Fe(OH)3¥2FeCl3+6NaCl;该反应中被还原的元素O,氧化产物是FeCl和Fe(OH)3(5)产生的刺激性气味的气体是氯气,反应的化学方程式为Na2O2+4HCl(浓)=2NaCl+Cl2↑+2H2O。