2022年全国联考精选卷(三)3理科综合答案

2022年全国联考精选卷(三)3理科综合答案,目前我们已经整理了2022年全国联考精选卷(三)3理科综合答案的各科答案和试卷,更多试卷答案请关注本答案网。

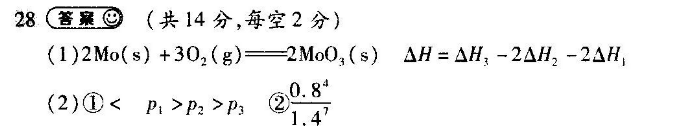


28(爸曩⑩(共14分,每空2分)(1)2Mo(s)+302(g)=2Mo3(s)△H=△H3-2△H2-2△H1(2)①
P2>p②21,4(3)①ab②=(4)0.125考什么Q命题人考查化学反应原理的综合应用么考0(1)①MS2(s)—Mo(s)+S2(g)AH1②S2(g)+202(g)=2S02(g)△H2③2MS2(s)+7O2(g)==2MoO3(s)+4SO2(g)△H3根据盖斯定律,③-2×②2-2×①,整理可得2Mo(s)+302(g)=2MoO3(s)△H=△H3-2△H2-2△H1。(2)①根据图象可以得出,随着温度升高,转化率逐渐降低,说明平衡逆向移动,根据平衡移动原理,可以得出ΔH3<0;根据方程式特点,增大压强,有利于平衡正向移动,氧气平衡转化率增大,所以有p1>P2>P3②根据三段式法,可以得出:2MoS(s)+7O2(g)←-2Mo0(s)+4s02(g初始量/mol转化量/mo2.0平衡量/mol(2.2×)(2.214(3)根据反应特点,若向正反应方向移动,气体质量会增加,气体的密度会增大,密度不变则达到平衡状态,a项正确;反应体系中仅有S种气体,气体的相对分子质量-直保持不变,不能通过相对分子质量判断是否达到平衡状态,b项正确;MoS2为固体,增加MoS2的量,平衡不移动,c项错误。②根据平衡常数不变以及平衡常数表达式,可以得出充入S2(g),再次达到平衡时,S2(g)浓度不变。(4)根据三段式法,可以得出S2(g)+202(g)=2S02(g)初始量/mo1.01.50转化量/mob平衡量/mol1.05-2x21.5-2x=0.5,则x=0.5,S2的反应速率为b(S2)=0.5mo/(2L2 min)=0. 125 moL(L. min

参考范文Then, clutching (5mff)the tin can, he headed for the shop. " Ihave the money. " he seriously told the owner. The man went to thewindow and fetched Reubens treasure, He wiped the dust of it andgently wrapped it in brown paper. Then he placed the parcel inReuhen's hands Racing home, Reuben burst through the front door.His mother was cleaning the kitchen stove. "Here, Mom! HereReuben exclaimed as he ran to her side. He placed a small box in herwork-roughened haHis mother unwrapped it carefully, to save the paper. A bluepaper box appeared, and she lifted the lid. In gold lettering on a pair ofred, lambskin gloves was the word Mother. And a card says,"It isMothers Day. We appreciate all the things that Mom does for usThank you! "His mother had never received such a gift; she had nogift except her wedding ring. Spccchlcss, she smiled sweetly andgathered her son into her arms, with tears running down her cheeks.Long live with this happy moment!